- Review
- Open access
- Published:
A review of animal models utilized in preclinical studies of approved gene therapy products: trends and insights
Laboratory Animal Research volume 40, Article number: 17 (2024)
Abstract
Scientific progress heavily relies on rigorous research, adherence to scientific standards, and transparent reporting. Animal models play a crucial role in advancing biomedical research, especially in the field of gene therapy. Animal models are vital tools in preclinical research, allowing scientists to predict outcomes and understand complex biological processes. The selection of appropriate animal models is critical, considering factors such as physiological and pathophysiological similarities, availability, and ethical considerations. Animal models continue to be indispensable tools in preclinical gene therapy research. Advancements in genetic engineering and model selection have improved the fidelity and relevance of these models. As gene therapy research progresses, careful consideration of animal models and transparent reporting will contribute to the development of effective therapies for various genetic disorders and diseases. This comprehensive review explores the use of animal models in preclinical gene therapy studies for approved products up to September 2023. The study encompasses 47 approved gene therapy products, with a focus on preclinical trials. This comprehensive analysis serves as a valuable reference for researchers in the gene therapy field, aiding in the selection of suitable animal models for their preclinical investigations.
Background
In the realm of gene therapy, a pivotal moment arrived with Paul Berg’s groundbreaking identification of the first recombinant DNA in 1972 [1]. This achievement not only marked a significant milestone but also served as the catalyst for a series of transformative breakthroughs in the field. Berg’s discovery fundamentally altered the landscape of genetic research, opening doors to novel therapeutic possibilities and paving the way for a new era of innovation and advancements in genetic engineering and gene therapy. Given the accelerated development of gene therapy products throughout the past century, this trend is anticipated to persist into the future [2], with a substantial portion of therapeutic inquiries focusing on preclinical investigations.
The principal objective of this comprehensive review article is to scrutinize and interpret preclinical research about gene therapy products that have garnered current approval and are presently administered to patients. This endeavour aspires to serve as an invaluable reference for researchers embarking on endeavours within the realm of gene therapy, seeking suitable animal models to facilitate their scientific undertakings.
Main text
The importance of preclinical studies in gene therapy clinical trials
Preclinical studies in the field of gene therapy play a pivotal role in advancing our understanding of genetic diseases and developing potential treatments. Additionally, all scientific progress and development are intricately intertwined with prior research endeavours. For scientific investigations to pave the way for significant advancements, they should embody three distinct attributes: (1) Adherence to Scientific Standards: The formulation and documentation of a study must strictly adhere to established scientific norms and guidelines. (2) Rigorous Parameterization in Animal Studies: In the realm of animal studies, meticulous attention to parameters is essential to ensure the reliability and validity of such investigations. (3) Transparent and Comprehensive Reporting: Researchers should exert utmost diligence in generating a report that is transparent, comprehensive, and credible in its entirety [3]. When these fundamental principles are observed in animal studies, they hold the potential to yield profound implications for the development of therapeutic products and our comprehension of disease pathophysiology. For instance, one of the most significant advantages of preclinical gene therapy studies is their ability to address diseases that lack effective avenues for investigation in human subjects, especially in the case of rare genetic diseases. In such instances, the creation of a standardized disease model not only facilitates the examination of all disease stages but also allows for elucidating the initial pathophysiological processes, even before the onset of clinical manifestations. Furthermore, some of these models elucidate genetic interrelationships, thereby uncovering potential modifier genes, a pursuit unfeasible within the confines of human subjects [4].
However, it is important to note that the success of preclinical gene therapy studies heavily relies on their adherence to scientific rigor, transparency, and meticulous reporting. The lack of these attributes can lead to issues such as irreproducibility and non-reproducibility, which hinder progress in the field [5,6,7,8,9,10,11,12]. This predicament often arises due to incomplete or inaccurate descriptions within research protocols, encompassing the allocation of animals among disparate study groups and the criteria underpinning the formation of said groups [11]. In addition to the formidable challenge of irreproducibility, another substantial hurdle resides in the discordance between the outcomes of animal studies and the results obtained from clinical trials. For example, clinical trials investigating stroke frequently yield results that diverge markedly from those generated in preclinical studies of the same condition. Root causes for this dissonance have been traced to the inability of any animal model to faithfully replicate the intricacies of human patients and the absence of robust, well-documented methodologies in the conduct of animal studies [13].
Considering the aforementioned quandaries, animal studies that yield congruent results in clinical trials can furnish superior methodologies for advancing subsequent investigations in related domains.
Animal models in gene therapy
The use of animal models in biomedical research, including gene therapy, is essential for gaining insights into complex biological systems and predicting the behaviour of interventions under specific conditions. These models serve as invaluable tools for researchers and can broadly be categorized into two primary functions: elucidating a system or process and predicting the behaviour of the target in question [14]. The concept of analogical reasoning, as initially introduced by Kant in the “Critique of Judgment”, posits that qualitative similarities between entities can be leveraged to forecast causal relationships, even in the presence of disparities [14]. With the advent of this concept, the application of models expanded across various scientific disciplines [15]. For instance, in the field of shipbuilding, scaled-down models are scrutinized to assess their designs, as hydrodynamics principles remain consistent, independent of scale. Conversely, in the biomedical sciences, including gene therapy, scalability lacks relevance [14] due to the diverse physical and behavioural attributes of organisms that impede such modelling. According to August Krogh’s principle, “For many problems, there is an animal on which it can be most conveniently studied” [16]. In biological sciences, the concept of analogy has supplanted scale, and its widespread applicability is attributed to the notion of “unity in diversity”, signifying fundamental relationships among organisms in terms of evolution and development [14]. Consequently, numerous animal models, notably laboratory animals such as mice, have been harnessed in diverse biological research endeavours.
Until 1980, mouse models predominantly comprised wild-type or spontaneously mutant species. Progress in fields such as chemotherapy and DNA-damaging agents owes much to the utilization of these animal models. Over the last four decades, a multitude of models catering to distinct objectives have emerged, thereby fostering advancements across various domains of biological science [17]. In recent decades, the significance of animal models has burgeoned due to the expansion of therapeutic product development, increased preclinical testing, and clinical trials. Foretelling therapeutic and safety outcomes in humans now constitutes the primary objective of experiments conducted before these products enter development, heavily contingent upon the judicious utilization of animal models [18].
The classification of animal models in the gene therapy era poses a formidable challenge, given their rapid proliferation and ongoing evolution. Moreover, diverse types of animal models each serve specific purposes, underscoring the critical importance of selecting the ideal model aligned with the research objectives. Meticulous model selection is imperative, as an erroneous choice can lead to inefficient resource allocation, ethical quandaries, and the generation of erroneous and unreliable scientific findings, potentially perpetuating inaccuracies in future experiments [19]. A 1985 NRC (National Research Council) report outlined various factors for the judicious selection of an appropriate animal model [14]. Paramount among these factors is the consideration of physiological and pathophysiological similarities between the model and the target of research. Additionally, the model’s capability to emulate desired conditions, such as disease-like states similar to those in the target (e.g., humans), warrants due consideration. Factors encompassing the model’s availability, size, lifespan, and others also play integral roles in this selection process [20]. Furthermore, individuals should be vigilant about potential mental and unconscious biases when selecting models, as familiarity or ease of use may unduly influence their choices [14].
One approach to mitigate the risk of inappropriate model selection involves the utilization of models specifically engineered for diverse conditions, such as genetically modified or humanized models closely mirroring human physiology in many aspects [21]. These models have witnessed substantial growth and find widespread application in research. Additionally, there are instances where a single animal model may prove inadequate to fulfill research objectives, necessitating the concurrent use of multiple models to ensure reliable and desired research outcomes [22]. Despite the multifaceted aspects elucidated concerning animal models, they are not the panacea for generalizing results and making biomedical predictions. It is essential to recognize that while alternatives to animal models have advanced significantly, they remain the sole practical choice for numerous experiments pertinent to human-related investigations. Numerous studies underscore that, notwithstanding their limitations, animal models persist as the primary resource for a multitude of experiments involving human subjects [14].
Preclinical gene therapy studies
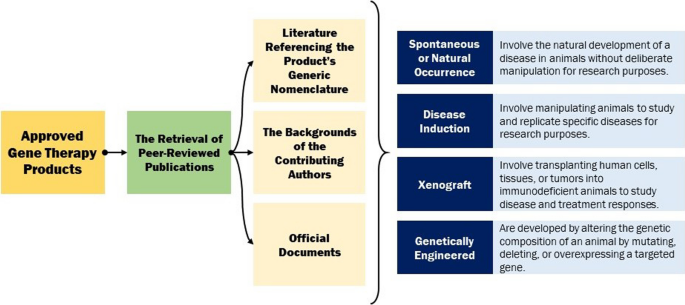
In this comprehensive analysis, a total of 47 approved gene therapy products, spanning from the inaugural approval of Vitravene to the latest sanctioned product as of September 2023, were meticulously scrutinized. The principal aim of this investigation entailed the retrieval of peer-reviewed publications about the preclinical trials of each product. This endeavour encompassed an extensive exploration through various means, including the pursuit of literature referencing the product’s generic nomenclature, the examination of the backgrounds of the contributing authors, and the scrutiny of pertinent articles from diverse sources. In some instances, official documents released by the regulatory bodies responsible for product approval were also consulted. In certain cases, regrettably, no accessible information concerning preclinical drug investigations was ascertainable. It is noteworthy that references cited within articles linked to the product under study were occasionally examined, even if the specific product was not explicitly mentioned therein. Furthermore, it should be noted that in several instances, multiple animal models were employed for the preclinical assessments. Additionally, a prevalent feature across the majority of these investigations was the reliance on common laboratory animals for safety and pharmacological studies, albeit without explicit specification.
The aggregate findings of this extensive inquiry yielded a corpus of 74 distinct animal models. The classification of animal models can be approached through various taxonomies, such as that delineated by Prabhakar, which delineates four primary categories: inbred strains, disease induction, xenograft, and genetically engineered models. Inbreeding has classically been used to obtain genetically homogeneous animals. Disease induction models are very commonly used to examine pathophysiology and drug development. Disease induction animal models involve manipulating animals to study and replicate specific diseases for research purposes. Xenograft animal models involve transplanting human cells, tissues, or tumour s into immunodeficient animals to study disease and treatment responses. Genetically engineered models are developed by altering the genetic composition of an animal by mutating, deleting, or overexpressing a targeted gene [23].
In alignment with the research objectives of this study, the “inbred” category within Prabhakar’s taxonomy was omitted, and a novel category denominated “spontaneous or natural occurrence” was introduced. Spontaneous or naturally occurring animal models involve the natural development of a disease in animals without deliberate manipulation for research purposes [24]. Consequently, the animal models under examination were categorized into four principal groups: disease induction, xenograft, genetically engineered, and spontaneous. In instances where the available information regarding the nature of the animal model utilized in the preclinical investigations of the product was indistinct or inadequately documented, such instances were classified as not applicable or N/A. It is pertinent to highlight that certain animal models were the product of mating between two animals with predetermined genetic attributes. In cases where the parentage of such models was naturally occurring, they were categorized as spontaneous. Conversely, if one or both progenitors had undergone genetic manipulation, their progeny were categorized as genetically engineered (Fig. 1).
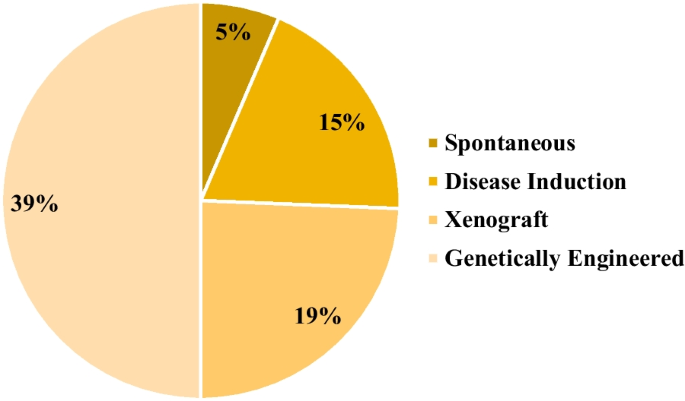
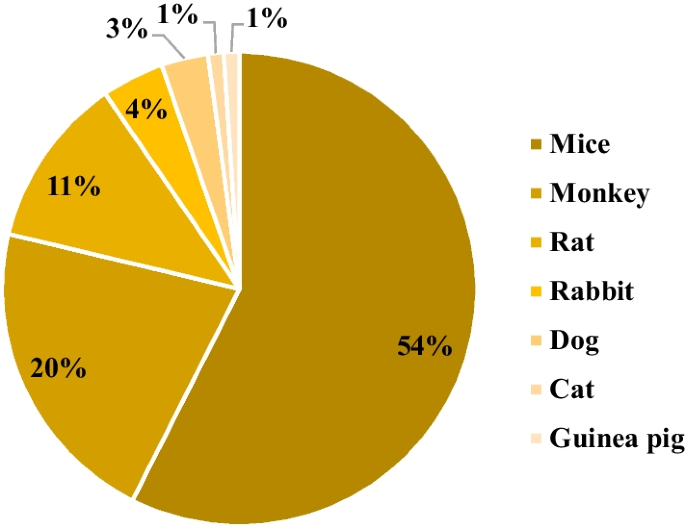
In the broader context, the analysis revealed that the genetically engineered category accounted for 39% of the identified animal models, followed by xenograft, disease induction, and spontaneous categories, with contributions of 19%, 15%, and 5%, respectively (Fig. 2). Additionally, 22% of the discerned animal models fell into the N/A category. Among the gamut of models scrutinized, mice emerged as the most frequently employed animal species, constituting 54% of the studies. Nonhuman primates claimed the second position, representing 20% of the investigated studies. Notably, other species were also incorporated into these investigations, including rats, rabbits, dogs, guinea pigs, and cats. A total of 6% of the studies did not involve the utilization of animal models (Fig. 3).
Furthermore, a granular examination of each category revealed distinctive utilization patterns. In the genetically engineered category, mice predominated, accounting for 79% of the animal species used, trailed by rats at 17%, and nonhuman primates at 7%. In the disease induction category, nonhuman primates emerged as the most frequently employed species, constituting 37% of the cases, with mice and rabbits equally sharing an 18% representation, while rats accounted for 27%. The xenograft category was overwhelmingly dominated by mice, comprising 93% of the animal species employed, with the residual 7% being nonhuman primates. In the spontaneous category, dogs featured 50% of the cases, followed by cats and mice, both with equal prevalence. Consequently, mice held sway in the genetically engineered and xenograft categories, while monkeys took precedence in the disease induction category, albeit with a caveat that 53% of the instances involving monkeys were categorized as uncertain, lacking substantive information regarding their role in the conducted studies. In the genetically engineered and disease induction categories, rats featured prominently (Table 1).
Utilization of animal models in preclinical investigations of cancer-related products
Among the 74 scrutinized studies, 18 were pertinent to cancer-related products (Table 2). Notably, animal models predominated as a fundamental component of these investigations, with the xenograft methodology being the principal mode of model generation, encompassing 61% of cancer-related animal models. In contrast, the remaining 39% comprised 6% attributed to genetic engineering, and 33% either lacked explicit animal model descriptions or adopted unspecified models. A significant proportion of 67% featured mice as the primary animal model species. Additionally, monkeys were employed in 11% of the studies related to cancer, while a singular study employed guinea pigs. Remarkably, a subset of three studies within this domain dispensed together the use of animal models.
Within the realm of preclinical appraisals about the aforementioned products, cell line-derived xenograft (CDX) models were notably prominent, particularly in the context of bone marrow cancers. It is worth highlighting that nude or immunodeficient mice receiving cancer cell grafts constituted the most frequently employed animal species. Moreover, the products Carvykti and Oncorine uniquely involved the utilization of monkeys and guinea pigs, respectively. In the context of lymphoma, associated with five distinct products, namely, Carteyva, Breyanzi, Tecartus, Kymriah and Yescarta, a conspicuous deficiency in efficient animal models for lymphoma was observed. Consequently, the relevant documentation articulated the absence of animal studies conducted for lymphoma [33, 34, 37, 38]. However, in the case of Breyanzi, a noteworthy exception emerged, wherein despite the initially stated lack of an efficient model for lymphoma, pharmacological investigations were conducted employing a Raji xenograft animal model [37]. This model was fashioned based on a distinctive framework devised by Buchsbaum and colleagues [38], characterized by specific attributes. A solitary instance within this purview featured the application of a conditional knockout mouse model, exclusively pertinent to Gendicine. It is pertinent to note that the spectrum of animal models for this particular drug extends more comprehensively, albeit with limited available information drawn from recent studies [25].
Utilization of animal models in preclinical investigations of nononcological products
Among the 74 scrutinized studies, 52 were directed toward nononcological products, encompassing a substantial proportion dedicated to genetic disorders (Table 3). In contrast to preclinical studies of cancer, 55% of the investigations in this section employed genetically engineered as the primary method for generating animal models. Induction techniques were applied in 17% of instances, while natural occurrences accounted for 8%, and xenografts represented 4%. The preeminent animal model employed in nononcological inquiries paralleled the cancer research sphere, with mice serving as the predominant choice, utilized in 53% of cases. In addition to mice, nonhuman primates featured more prominently, constituting 19% of the studies. Rats were also frequently enlisted, contributing to 16% of the animal models in this category. Other species enlisted in this realm comprised rabbits (4%), dogs (4%), and cats (2%).
Significantly, a substantial portion of the models within this category was rooted in genetically engineered models. Such models in preclinical studies emanated from two principal avenues: procurement from commercial laboratories or in-house generation by researchers. Moreover, in some investigations, the primary model served as a foundation, inheriting genetic alterations from other genetically engineered models, or the foundational disease model emerged through the mating of two distinct genetically modified models (as observed in the EMA (European Medicines Agency) document for Rovtavian) [83]. Additionally, mice, rats, and nonhuman primates were the prevalent species subjected to genetic engineering, each bearing unique attributes pertinent to specific research objectives. In the majority of cases, animals exhibited specific genetic aberrations, albeit certain exceptions involved the use of highly immunodeficient mice, as exemplified in the Skysona study [79].
Beyond genetic engineering, induction, natural occurrences, and xenograft methods also found applicability within this category. The induction methodology was multifariously employed to replicate disorders such as adult familial chylomicronemia syndrome and ischemia or arteritis, accomplished through specialized dietary regimens or surgical procedures. Rat and monkey species constituted the primary subjects of experimentation within this domain, although mice and rabbits were sporadically incorporated. In the natural occurrence category, dogs emerged as the primary species of choice, with a solitary instance of cat utilization documented [44]. A noteworthy case, pertinent to the Libmeldy product, involved the creation of an animal model through the interbreeding of two species with naturally occurring disorders [72]. In contrast, the adoption of xenograft techniques was relatively limited in this category, with only three investigations resorting to this method. Notably, Vyjuvek and Strimvelis product research incorporated the grafting of cells bearing disease-related defects into severely immunodeficient mice [49, 86]. The study associated with the Zalmoxis product similarly employed this method to augment the immune system following the grafting of hematopoietic stem cells.
Of the 74 examined studies, 4 studies were concerned with products about infectious diseases (Table 4). In these infectious disease inquiries, the predominant animal models of choice encompassed nonhuman primates and rabbits, primarily induced through techniques such as induction.
Trending approaches in the development of animal models for investigative research
The preeminent method for establishing animal models in cancer research is notably the xenograft approach. Within the purview of xenograft studies, the CDX method stands as the ubiquitous choice. Indeed, the advent of CDX models followed the discernment of metastatic tendencies and their intricate association with the site of tumour cell inoculation in laboratory animals. These models hinge upon the subcutaneous or intravenous injection of human cancer cells into immunocompromised mice, a procedure readily achievable within the confines of a laboratory setting. CDX models have exhibited marked efficacy in the development of cytotoxic cancer therapies [92]. However, they have proven less efficacious when utilized for drugs targeting specific proteins [93]. The utility of CDX models is contingent upon the specific objectives of a study. Among their advantages are their suitability for investigating underlying mechanisms, cost-effectiveness, and expeditious development. Additionally, they prove instrumental in the assessment of nonspecific cytotoxic agents. Conversely, their limitations encompass the lack of heterogeneity within models generated through this method, the inability to undertake immunological investigations utilizing these models, and their sole composition of cancer cells, bereft of the rich tumour microenvironment [94, 95]. Notwithstanding these drawbacks, CDX models remain the favoured choice for preclinical studies and find extensive use in the majority of scrutinized cases. Furthermore, their utilization in diverse research domains has witnessed a substantial upsurge, underscoring their enduring popularity [96].
It is imperative to also consider the emergence of patient-derived xenograft (PDX) models, which ameliorate the constraints intrinsic to other methodologies, yielding more efficacious animal models. PDX models preserve not only the tumour microenvironment but also the heterogeneity and mutagenic characteristics of tumours. Furthermore, they facilitate the study of metastasis, with the generated model serving as a suitable biological surrogate. However, it is noteworthy that PDX models can only be generated in severely immunocompromised mice, and their efficiency exhibits variability, rendering them less suitable for early-stage cancer research [97, 98]. Thus, a judicious evaluation of the facets of preclinical studies can lead to the adoption of novel and more efficacious models, enhancing the quality of such investigations.
Additionally, as previously mentioned, genetic manipulation has emerged as the preeminent method in investigations of nononcological diseases. This approach affords the potential for creating models that closely mirror the characteristics of the original disease. Recent years have witnessed a substantial proliferation in the usage of such models, attributed to the advent of engineered endonucleases, which enable precise and efficient genome editing [99,100,101]. The key step in genome editing is the induction of site-specific double-strand breaks (DSBs) by engineered endonucleases that are subsequently corrected by one of two competing DNA repair pathways, nonhomologous end-joining (NHEJ) and homology-directed repair (HDR) [102]. Recent advances in genome editing technologies reflect the rapid development of engineered endonucleases, including zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeat (CRISPR) systems [103]. These endonucleases endow genome editing with two pivotal attributes: 1) the capacity to selectively recognize specific target sequences and 2) a high degree of compatibility for the placement of specified sequences [104]. Predominantly, the genetic modifications affecting the animal models under scrutiny are knockouts. For instance, in a preclinical study centred on Glybera, a product related to familial lipoprotein lipase deficiency, mice with knockout genomic regions linked to lipoprotein lipase were employed [44]. Similarly, in the context of the Rovtavian product, which is associated with hemophilia A, knockout mice have been instrumental [83]. Such instances abound in the corpus of examined research.
The primary objective of knockout is to supplant a specific genomic segment with one that is either nonfunctional, modified, or irrelevant. This substitution can precipitate alterations in the phenotype of the animal model, thereby manifesting unique disease characteristics. The development of these models represents a watershed moment in the realm of animal models and therapeutic product development. The field has witnessed a plethora of advances that permit increasingly specific and temporally controlled genetic manipulations, in addition to confining mutations to designated tissues [105]. Notwithstanding these commendable strides, challenges persist in the handling of these models. For instance, target genes may not always be amenable to genetic manipulation, and genetic editing in these models is a complex endeavour that may engender metabolic perturbations within the animal’s pathways, precipitating phenotypic anomalies [106]. Nonetheless, the usage of genetically modified animal models is burgeoning, with the advent of novel technologies that hold the potential to ameliorate the limitations of prior models, thereby engendering models of greater aptitude than their predecessors.
Trending species in the animal models for investigative research
As indicated by the findings of this study, the preclinical investigation of gene therapy products predominantly employs the mouse model, which stands as the most prevalent species of choice. Furthermore, upon closer scrutiny, it becomes evident that mice are extensively employed in the development of genetically modified animal models. The utilization of mice as an animal model boasts several merits, including cost-effectiveness in maintenance. In addition, their rapid reproduction rate and comparatively short lifespan render them ideal for genetic inquiries. Significantly, mice exhibit an estimated genetic similarity to humans in the range of 99% [107]. Furthermore, the extensive research conducted on their genetic resources, which are publicly accessible [108, 109], underscores their prominence as a preferred model for conducting preclinical investigations.
Consequently, following mice, nonhuman primates emerge as the second most utilized species in the research endeavours under review. Phylogenetically, nonhuman primates share the closest genetic proximity to humans and find widespread application in diverse domains, encompassing psychiatric, metabolic, reproductive, and immunological studies [52]. In the specific context of the studies under consideration, nonhuman primates were predominantly deployed for disease induction purposes. However, some instances featured their deployment as noncompliant subjects, likely chosen for safety and toxicity assessments. It is worth noting that despite the marked desirability of employing this species, limitations such as restricted availability, associated expenses, and ethical concerns regarding genetic manipulation serve as constraining factors [110].
Within the third category of animal models, rats were also included. Rats serve as apt animal models extensively employed in the examination of physiology and pathophysiology, and they constitute a suitable choice for evaluating the efficacy and toxicity of clinical trials [111,112,113]. In the studies scrutinized, rats were most frequently employed in genetic manipulations.
Last, it is noteworthy that dogs were solely featured in the studies under consideration as models with naturally occurring traits. Specifically, hereditary diseases in dogs, classified as naturally occurring, bear the highest clinical resemblance to human diseases [114]. This congruence has engendered substantial demand for the use of dogs in these particular contexts.
Conclusions
The selection of an appropriate animal model constitutes a pivotal and fundamental step in the execution of animal studies, particularly within the domain of preclinical research. This selection process necessitates strict adherence to established scientific criteria and standards, as it holds the key to attaining optimal outcomes not only in the present investigation but also in subsequent research endeavours. An effective strategy for model selection involves recourse to prior studies that have traversed all requisite phases, culminating in the approval of resultant products. By doing so, one can confidently employ the chosen animal model and extend the generalizability of its findings to forthcoming investigations. Moreover, this retrospective approach enables the identification of successful methodologies for generating animal models and the identification of species suitable for the intended research purposes.
In the context of the current study, we focused on the examination of animal models employed in preclinical assessments of gene therapy products. Our findings have illuminated that the xenograft methodology, predominantly implemented through the CDX technique, stands as the most prevalent approach in preclinical studies about cancer therapeutics. Furthermore, in the realm of generating animal models for diverse pathologies, with a particular emphasis on genetic disorders, genetic manipulation emerges as the predominant technique, particularly in the creation of knockout models. Within this landscape, mice and nonhuman primates have emerged as the two most frequently utilized species.
Notably, recent trends underscore a discernible upswing in the utilization of mice and genetic manipulation methodologies as we approach the contemporary era. It is imperative not to overlook the transformative potential inherent in emerging technologies for the creation of these animal models, as the incorporation of state-of-the-art innovations undoubtedly holds promise for the generation of models of superior quality and fidelity.
Availability of data and materials
All datasets on which the conclusions of this article rely are presented within the article. No additional data repositories are required as all relevant data can be found within the manuscript itself. We have taken care to ensure that the data is easily accessible to readers in the main paper.
Abbreviations
- NRC:
-
National research council
- CDX:
-
Cell line-derived xenograft
- EMA:
-
European medicines agency
- PDX:
-
Patient-derived xenograft
- DSBs:
-
Double-strand breaks
- NHEJ:
-
Nonhomologous end-joining
- HDR:
-
Homology-directed repair
- ZFNs:
-
Zinc finger nucleases
- TALENs:
-
Transcription activator-like effector nucleases
- CRISPR:
-
Clustered regularly interspaced short palindromic repeat
References
Shchaslyvyi AY, Antonenko SV, Tesliuk MG, Telegeev GD. Current state of human gene therapy: approved products and vectors. Pharmaceuticals. 2023;16(10):1416.
Arabi F, Mansouri V, Ahmadbeigi N. Gene therapy clinical trials, where do we go? An overview. Biomed Pharmacother. 2022;153: 113324.
Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–91.
Guénet JL. Animal models of human genetic diseases: do they need to be faithful to be useful? Mol Genet Genomics. 2011;286:1–20.
Begley CG. Raising standards for preclinical research. Evid Based Preclin Med. 2014;1: e00003.
Hess KR. Statistical design considerations in animal studies published recently in cancer research. Cancer Res. 2011;71:625.
Kilkenny C, Parsons N, Kadyszewski E, Festing MFW, Cuthill IC, Fry D, et al. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLoS ONE. 2009;4: e7824.
Moher D, Simera I, Schulz KF, Hoey J, Altman DG. Helping editors, peer reviewers and authors improve the clarity, completeness and transparency of reporting health research. BMC Med. 2008;6:13.
Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov. 2011;10:712.
Sena E, van der Worp HB, Howells D, Macleod M. How can we improve the pre-clinical development of drugs for stroke? Trends Neurosci. 2007;30:433–9.
Steward O, Popovich PG, Dietrich WD, Kleitman N. Replication and reproducibility in spinal cord injury research. Exp Neurol. 2012;233:597–605.
Van der Worp HB, Macleod MR. Preclinical studies of human disease: time to take methodological quality seriously. J Mol Cell Cardiol. 2011;51:449–50.
Fisher M. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke Stroke Therapy Academic Indus Roundtable. 1999;30:2752–8.
Wall RJ, Shani M. Are animal models as good as we think? Theriogenology. 2008;69:2–9.
Popper KR. The logic of scientific discovery. Central works of philosophy v4: Twentieth Century: Moore to Popper. 2015. pp.262–286.
Krebs HA. The August Krogh principle: “For many problems there is an animal on which it can be most conveniently studied.” J Exp Zool. 1975;194:221–6.
Lee H. Genetically engineered mouse models for drug development and preclinical trials. Biomol Ther. 2014;22:267–74.
Pehlivanovic B, Smajic NZ, Belma P, Dina F, Emina A, Nermina Ž, et al. Animal Models in Modern Biomedical Research. Eur J Pharm Med Res. 2019
Connors TA. Animal models in toxicology. J Pharm Pharmacol. 1993;45:1015.
Mukherjee P, Roy S, Ghosh D, Nandi SK. Role of animal models in biomedical research: a review. Lab Anim Res. 2022;38:1–18.
Simmons D. The Use of Animal Models in Studying Genetic Disease. Nature Education. 2008;1–10.
Van Dam D, De Deyn PP. Animal models in the drug discovery pipeline for Alzheimer’s disease. Br J Pharmacol. 2011;164:1285–300.
Prabhakar S. Translational research challenges: finding the right animal models. J Investig Med. 2012;60:1141–6.
Hansen K, Khanna C. Spontaneous and genetically engineered animal models: Use in preclinical cancer drug development. Eur J Cancer. 2004;40:858–80.
Engelmann D, Pützer BM. Emerging from the shade of p53 mutants: N-terminally truncated variants of the p53 family in EMT signaling and cancer progression. Sci Signal. 2014;7:re9.
Li Y, Li B, Li CJ, Li LJ. Key points of basic theories and clinical practice in rAd-p53 (Gendicine™) gene therapy for solid malignant tumors. Expert Opin Biol Ther. 2015;15:437–54.
Liang M. Oncorine, the world first oncolytic virus medicine and its update in China. Curr Cancer Drug Targets. 2018;18:171–6.
Gordon EM, Hall FL. Rexin-G, a targeted genetic medicine for cancer. Expert Opin Biol Ther. 2010;10:819–32.
Kohlhapp FJ, Kaufman HL. Molecular pathways: Mechanism of action for talimogene laherparepvec, a new oncolytic virus immunotherapy. Clin Cancer Res. 2016;22:1048–54.
Liu BL, Robinson M, Han ZQ, Branston RH, English C, Reay P, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Therapy. 2003;10:292–303.
Elsallab M, Levine BL, Wayne AS, Abou-el-enein M, Abou-el- PM, Berlin U, et al. Before and after marketing authorisation. Lancet Oncol. 2020;21:104–16.
Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–64.
SUMMARY OF PRODUCT CHARACTERISTICS—Yescarta. In: European Medicines Agency. 2022. https://www.ema.europa.eu/en/documents/product-information/yescarta-epar-product-information_en.pdf.
SUMMARY OF PRODUCT CHARACTERISTICS—Tecartus. In: European Medicines Agency. 2022. https://www.ema.europa.eu/en/documents/product-information/tecartus-epar-product-information_en.pdf.
Assessment report of Abecma. In: European Medicines Agency. 2021. https://www.ema.europa.eu/en/documents/assessment-report/abecma-epar-public-assessment-report_en.pdf.
Maldonado-Pérez N, Tristán-Manzano M, Justicia-Lirio P, Martínez-Planes E, Muñoz P, Pavlovic K, et al. Efficacy and safety of universal (TCRKO) ARI-0001 CAR-T cells for the treatment of B-cell lymphoma. Front Immunol. 2022;13:1–17.
Assessment report of Breyanzi. In: European Medicines Agency. 2022. https://www.ema.europa.eu/en/documents/assessment-report/breyanzi-epar-public-assessment-report_en.pdf
Buchsbaum DJ, Wahl RL, Normolie DP, Kaminski MS. Therapy with unlabeled and 131I-labeled pan-B-cell monoclonal antibodies in nude mice bearing Raji Burkitt’s Lymphoma Xenografts. Cancer Res. 1992;52:6476–81.
Cheema TA, Wakimoto H, Fecci PE, Ning J, Kuroda T, Jeyaretna DS, et al. Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model - Supporting Information. Proc Natl Acad Sci. 2013;110:12006–11.
Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci USA. 2001;98:6396–401.
Summary Basis for Regulatory Action—ADSTILADRIN. In: U.S. Food and Drug Administration. 2022. https://www.fda.gov/media/164532/download.
Seckinger A, Delgado JA, Moser S, Moreno L, Neuber B, Grab A, et al. Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer Cell. 2017;31:396–410.
Bozo IY, Drobyshev AY, Redko NA, Komlev VS, Isaev AA, Deev RV. Bringing a gene-activated bone substitute into clinical practice: from bench to bedside. Front Bioeng Biotechnol. 2021;9:1–14.
Bryant LM, Christopher DM, Giles AR, Hinderer C, Rodriguez JL, Smith JB, et al. Lessons learned from the clinical development and market authorization of Glybera. Hum Gene Ther Clin Dev. 2013;24:55–64.
Ross CJD, Twisk J, Meulenberg JM, Liu G, Van Den Oever K, Moraal E, et al. Long-term correction of murine lipoprotein lipase deficiency with AAV1-mediated gene transfer of the naturally occurring LPL S447X beneficial mutation. Hum Gene Ther. 2004;15:906–19.
Hair P, Cameron F, McKeage K. Mipomersen sodium: first global approval. Drugs. 2013;73:487–93.
Lim KRQ, Maruyama R, Yokota T. Eteplirsen in the treatment of Duchenne muscular dystrophy. Drug Des Dev Ther. 2017;11:533–45.
Singh NN, Howell MD, Androphy EJ, Singh RN. How the discovery of ISS-N1 led to the first medical therapy for spinal muscular atrophy. Gene Ther. 2017;24:520–6.
Aiuti A, Roncarolo MG, Naldini L. Gene therapy for ADA-SCID, the first marketing approval of an ex vivo gene therapy in Europe: paving the road for the next generation of advanced therapy medicinal products. EMBO Mol Med. 2017;9:737–40.
Ferrari G, Rossini S, Giavazzi R, Maggioni D, Nobili N, Soldati M, et al. An in vivo model of somatic cell gene therapy for human severe combined immunodeficiency. Science. 1991;251:1363–6.
Assessment report of Zalmoxis. In: European Medicines Agency. 2016. https://www.ema.europa.eu/en/documents/assessment-report/zalmoxis-epar-public-assessment-report_en.pdf.
Lee H, Choi K, Kim H, Kim D, Lee Y, Lee B, et al. INVOSSA-K induces an anti-inflammatory environment in a rat mia model via macrophage polarization. Osteoarthritis Cartilage. 2018;26:S125.
CharlesRiver. Osteoarthritis Model. Encyclopedia of Pain. 2013. pp. 2558–2558.
Ciulla TA, Hussain RM, Berrocal AM, Nagiel A. Voretigene neparvovec-rzyl for treatment of RPE65-mediated inherited retinal diseases: a model for ocular gene therapy development. Expert Opin Biol Ther. 2020;20:565–78.
Butler JS, Chan A, Costelha S, Fishman S, Willoughby JLS, Borland TD, et al. Preclinical evaluation of RNAi as a treatment for transthyretin-mediated amyloidosis. Amyloid. 2016;23:109–18.
Ackermann EJ, Guo S, Benson MD, Booten S, Freier S, Hughes SG, et al. Suppressing transthyretin production in mice, monkeys and humans using 2nd-Generation antisense oligonucleotides. Amyloid. 2016;23:148–57.
Benson MD, Kluve-Beckerman B, Zeldenrust SR, Siesky AM, Bodenmiller DM, Showalter AD, et al. Targeted suppression of an amyloidogenic transthyretin with antisense oligonucleotides. Muscle Nerve. 2006;33:609–18.
Suda H, Murakami A, Kaga T, Tomioka H, Morishita R. Beperminogene perplasmid for the treatment of critical limb ischemia. Expert Rev Cardiovasc Ther. 2014;12:1145–56.
Taniyama Y, Morishita R, Hiraoka K, Aoki M, Nakagami H, Yamasaki K, et al. Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat diabetic hind limb ischemia model. Circulation. 2001;104:2344–50.
Van Putten M, Hulsker M, Young C, Nadarajah VD, Heemskerk H, Van Der Weerd L, et al. Low dystrophin levels increase survival and improve muscle pathology and function in dystrophin/utrophin double-knockout mice. FASEB J. 2013;27:2484–95.
Servais L, Mercuri E, Straub V, Guglieri M, Seferian AM, Scoto M, et al. Long-term safety and efficacy data of golodirsen in ambulatory patients with duchenne muscular dystrophy amenable to exon 53 skipping: a first-in-human, multicenter, two-part, open-label, phase 1/2 trial. Nucleic Acid Ther. 2022;32:29–39.
D’Erasmo L, Gallo A, Di Costanzo A, Bruckert E, Arca M. Evaluation of efficacy and safety of antisense inhibition of apolipoprotein C-III with volanesorsen in patients with severe hypertriglyceridemia. Expert Opin Pharmacother. 2020;00:1675–84.
Graham MJ, Lee RG, Bell TA, Fu W, Mullick AE, Alexander VJ, et al. Antisense oligonucleotide inhibition of apolipoprotein c-iii reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ Res. 2013;112:1479–90.
Assessment report of Zolgensma. In: European Medicines Agency. 2020. https://www.ema.europa.eu/en/documents/assessment-report/zolgensma-epar-public-assessment-report_en.pdf.
SUMMARY OF PRODUCT CHARACTERISTICS—Zolgensma. In: European Medicines Agency. 2022. https://www.ema.europa.eu/en/documents/product-information/zolgensma-epar-product-information_en.pdf.
SUMMARY OF PRODUCT CHARACTERISTICS—Zynteglo. In: European Medicines Agency. 2022. https://www.ema.europa.eu/en/documents/product-information/zynteglo-epar-product-information_en.pdf
Scott LJ. Givosiran: first approval. Drugs. 2020;80:335–9.
SUMMARY OF PRODUCT CHARACTERISTICS—Givlaari. In: European Medicines Agency. 2020. https://www.ema.europa.eu/en/documents/product-information/givlaari-epar-product-information_en.pdf.
Chan A, Liebow A, Yasuda M, Gan L, Racie T, Maier M, et al. Preclinical development of a subcutaneous ALAS1 RNAi therapeutic for treatment of hepatic porphyrias using circulating RNA quantification. Mol Ther Nucleic Acids. 2015;4: e263.
Assessment report of Leqvio. In: European Medicines Agency. 2020. https://www.ema.europa.eu/en/documents/assessment-report/leqvio-epar-public-assessment-report_en.pdf.
Fitzgerald K, White S, Borodovsky A, Bettencourt BR, Strahs A, Clausen V, et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med. 2017;376:41–51.
Biffi A, De Palma M, Quattrini A, Del Carro U, Amadio S, Visigalli I, et al. Correction of metachromatic leukodystrophy in the mouse model by transplantation of genetically modified hematopoietic stem cells. J Clin Investig. 2004;113:1118–29.
Messina M, Gissen P. Atidarsagene autotemcel for metachromatic leukodystrophy. Drugs of Today. 2023;59:63–70.
Liebow A, Li X, Racie T, Hettinger J, Bettencourt BR, Najafian N, et al. An investigational RNAi therapeutic targeting glycolate oxidase reduces oxalate production in models of primary hyperoxaluria. J Am Soc Nephrol. 2017;28:494–503.
Scott LJ, Keam SJ. Lumasiran: first approval. 2021;277–82.
Shimatsu Y, Katagiri K, Furuta T, Nakura M, Tanioka Y, Yuasa K, et al. Canine X-linked muscular dystrophy in Japan (CXMDJ). Exp Anim. 2003;52:93–7.
Yucel N, Chang AC, Day JW, Rosenthal N, Blau HM. Humanizing the mdx mouse model of DMD: the long and the short of it. npj Regenerative Medicine. 2018;3.
Brolin C, Shiraishi T. Antisense mediated exon skipping therapy for duchenne muscular dystrophy (DMD). Artif DNA PNA XNA. 2011;2:6–15.
Assessment report of Skysona. In: European Medicines Agency. 2021. https://www.ema.europa.eu/en/documents/assessment-report/skysona-epar-public-assessment-report_en.pdf.
Summary Basis for Regulatory Action—SKYSONA. In: U.S. Food and Drug Administration. 2022. https://www.fda.gov/media/162098/download.
SUMMARY OF PRODUCT CHARACTERISTICS—Hemgenix. In: European Medicines Agency. 2023. https://www.ema.europa.eu/en/documents/product-information/hemgenix-epar-product-information_en.pdf.
Summary Basis for Regulatory Action—HEMGENIX. In: U.S. Food and Drug Administration. 2022. https://www.fda.gov/media/164094/download.
Assessment report of Roctavian. In: European Medicines Agency. 2022. https://www.ema.europa.eu/en/documents/assessment-report/roctavian-epar-public-assessment-report_en.pdf.
Assessment report of Upstaza. In: European Medicines Agency. 2022. https://www.ema.europa.eu/en/documents/assessment-report/upstaza-epar-public-assessment-report_en.pdf.
Summary Basis for Regulatory Action—ELEVIDYS. In: U.S. Food and Drug Administration. 2023. https://www.fda.gov/media/169746/download.
Gurevich I, Agarwal P, Zhang PP, Dolorito JA, Oliver S, Liu H, et al. In vivo topical gene therapy for recessive dystrophic epidermolysis bullosa: a phase 1 and 2 trial. Nat Med. 2022;28:780–8.
DRAFT U.S. PACKAGE INSERT—Vitravene. In: U.S. Food and Drug Administration. 1998. https://www.accessdata.fda.gov/drugsatfda_docs/nda/98/20961_Vitravene_prntlbl.pdf.
Dix RD, Cousins SW. AIDS-related cytomegalovirus retinitis: Lessons from the laboratory. Curr Eye Res. 2004;29:91–101.
Oh JJ, Carter JJ, Dix RD. A mouse model that mimics aids-related cytomegalovirus retinitis: Insights into pathogenesis. Pathogens. 2021;10:1–15.
Khehra N, Mahtani A, Rehman O, Jaferi U, Kipker N. Elasomeran (mRNA1273) Vaccine: The Journey from Preclinical Research to Clinical Trials, Authorization, and FDA Approval. 2022.
Khehra N, Padda I, Jaferi U, Atwal H, Narain S, Parmar MS. Tozinameran (BNT162b2) Vaccine: The Journey from Preclinical Research to Clinical Trials and Authorization. AAPS PharmSciTech. 2021. 172.
Day CP, Merlino G, Van Dyke T. Preclinical mouse cancer models: a maze of opportunities and challenges. Cell. 2015;163:39–53.
Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84:1424–31.
Ajith A, Mulloy LL, Musa MA, Bravo-Egana V, Horuzsko DD, Gani I, et al. Humanized mouse model as a novel approach in the assessment of human allogeneic responses in organ transplantation. Front Immunol. 2021;12: 687715.
Georges LMC, De Wever O, Galván JA, Dawson H, Lugli A, Demetter P, et al. Cell line derived xenograft mouse models are a suitable in vivo model for studying tumor budding in colorectal cancer. Front Med. 2019;6:139.
Abdolahi S, Ghazvinian Z, Muhammadnejad S, Saleh M, Asadzadeh Aghdaei H, Baghaei K. Patient-derived xenograft (PDX) models, applications and challenges in cancer research. J Transl Med. 2022;20(1):206.
Jung J, Seol HS, Chang S. The generation and application of patient-derived xenograft model for cancer research. Cancer Res Treat. 2018;50:1–10.
Pearson AT, Finkel KA, Warner KA, Nör F, Tice D, Martins MD, et al. Patient-derived xenograft (PDX) tumors increase growth rate with time. Oncotarget. 2016;7:7993–8005.
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 1979;2013(339):819–23.
Gaj T, Gersbach CA, Barbas CF. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31:397–405.
Shao M, Xu TR, Chen CS. The big bang of genome editing technology: development and application of the CRISPR/Cas9 system in disease animal models. Dongwuxue Yanjiu. 2016;37:191–204.
Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. 2014;32:347–50.
Lee JG, Sung YH, Baek IJ. Generation of genetically-engineered animals using engineered endonucleases. Arch Pharm Res. 2018;41:885–97.
Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;11:636–46.
Majzoub JA, Muglia LJ. Knockout Mice. N Engl J Med. 1996;334:904–7.
Houdebine LM. Transgenic animal models in biomedical research. Methods Mol Biol. 2007. p. 163–202.
Boguski MS. The mouse that roared. Nature. 2002;420:515–6.
Eppig JT. Mouse genome informatics (MGI) resource: genetic, genomic, and biological knowledgebase for the laboratory mouse. ILAR J. 2017;58:17–41.
Paigen K. A miracle enough: the power of mice. Nat Med. 1995;1:215–20.
Chan AWS. Progress and prospects for genetic modification of nonhuman primate models in biomedical research. ILAR J. 2013;54:211–23.
Aitman TJ, Critser JK, Cuppen E, Dominiczak A, Fernandez-Suarez XM, Flint J, et al. Progress and prospects in rat genetics: a community view. Nat Genet. 2008;40:516–22.
Jacob HJ. Functional genomics and rat models. Genome Res. 1999;9:1013–6.
Jacob HJ, Kwitek AE. Rat genetics: attaching physiology and pharmacology to the genome. Nat Rev Genet. 2002;3:33–42.
Shearin AL, Ostrander EA. Leading the way: canine models of genomics and disease. DMM Dis Models Mech. 2010;3:27–34.
Acknowledgements
We would like to acknowledge that there are no specific individuals or organizations to acknowledge for their contributions to this research.
Funding
The article lacks any sources of funding that require declaration.
Author information
Authors and Affiliations
Contributions
PS contributed to data collection, analysis, and interpretation and also drafted the article. VM revised it critically for important intellectual content. NA contributed to the conception and design of the study. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no financial or non-financial competing interests related to this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Soufizadeh, P., Mansouri, V. & Ahmadbeigi, N. A review of animal models utilized in preclinical studies of approved gene therapy products: trends and insights. Lab Anim Res 40, 17 (2024). https://doi.org/10.1186/s42826-024-00195-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42826-024-00195-6